Abstract
-
The groundwater of the Dhuleil, Halabat, and Samra areas of Jordan was studied to determine reasons for the build up of salinity over time.
The quality of the groundwater in the region deteriorated after more than two decades of use for irrigation. Chemical analysis of the groundwater showed extremely high concentrations of salts far exceeding the acceptable limit for various purposes as given in the Jordanian Water Quality Guideline.
ArcView "Spatial Analyst" proved to be an excellent tool for analyzing and displaying the spatial extent of groundwater contamination. The results of this study show that the return flow of irrigation and infiltration of treated waste water into the groundwater are the main reasons for the salination problem rather than the invasion of saline water from deep layers due to over-pumping.
Introduction
-
The study area, is part of the Amman-Zarqa basin in Jordan. The investigated area is approximately 450 Km2 and consist of three districts: Dhuleil, Halabat, and Samra.
The climate is considered arid where the average amount of precipitation, evaporation, relative humidity, and average temperature is 150 mm/y, 1500 mm/year, 55%, and 17.4 oC respectively for a period of 19 years (Water Authority of Jordan, Data-files).
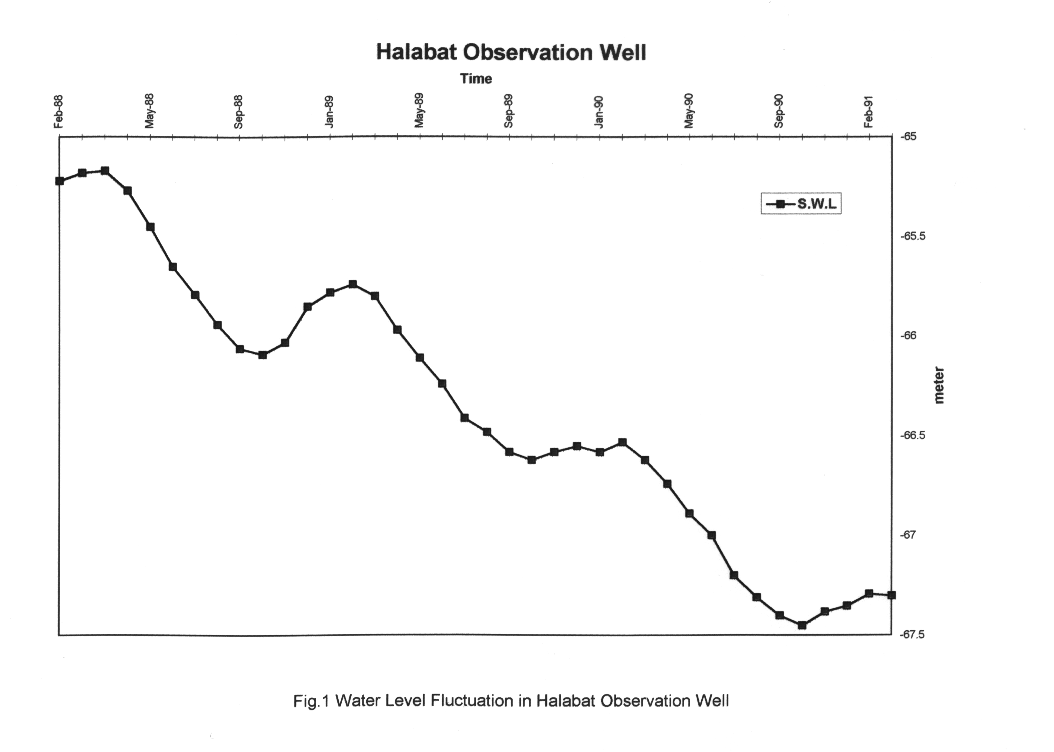
Agriculture development began in the middle of the nineteen sixties and seventies when a number of wells were drilled by the private sector for irrigation purposes. Groundwater was the only available source for water supply and irrigation in the region, therefore groundwater was abstracted heavily to meet the need for expansion in the agricultural lands. This practice increased the rate of pumping in the wells causing a general decline in the water level. The draw-down in some wells declined from 0.14-0.41 m in the early seventies up to 19.45 - 27.88 m in the early nineties. Fig.1 shows a decline of 2 meters in water level for the Halabat observation well over the period February 1988 to February 1991.
The excessive use of fertilizers added to the soil to improve fertility and crop yield significantly decreased the quality of groundwater. These chemical fertilizers dissolved in the irrigation water and ended up in the aquifer. Therefore, the quality of groundwater was affected and this resulted in a major increase in cations and anions in the water chemistry. For the majority of groundwater wells, the quality shifted from fresh water (salinity less than 500 mg/l) to brackish water (salinity higher than 1500 mg/l).
In 1983 the Khalideh Dam was built in the Dhuleil area with a 1 million cubic meter (MCM) capacity, the purpose of this dam was to store flood water in winter time (October to April) and later inject this stored water back into a groundwater well built specifically for this purpose. The expectation of this artificial recharge project was that it would improve the quality of groundwater.
In 1985, the Alsamra Wastewater Treatment Plant (AWTP) was built in the Samra area with a capacity of 20 MCM to treat the domestic and industrial water of Greater Amman (approximately one million inhabitant). The groundwater of the shallow aquifer downstream of the AWTP became highly contaminated especially with nitrate.
Site Characteristics
- The geological formation outcropping in the area are mainly carbonate rocks with thick flows of basalt (Fig.2). The carbonate rock formations range in age from Turonian to Campanian, while the basalt ranges from Oligocene to Pleistocene.
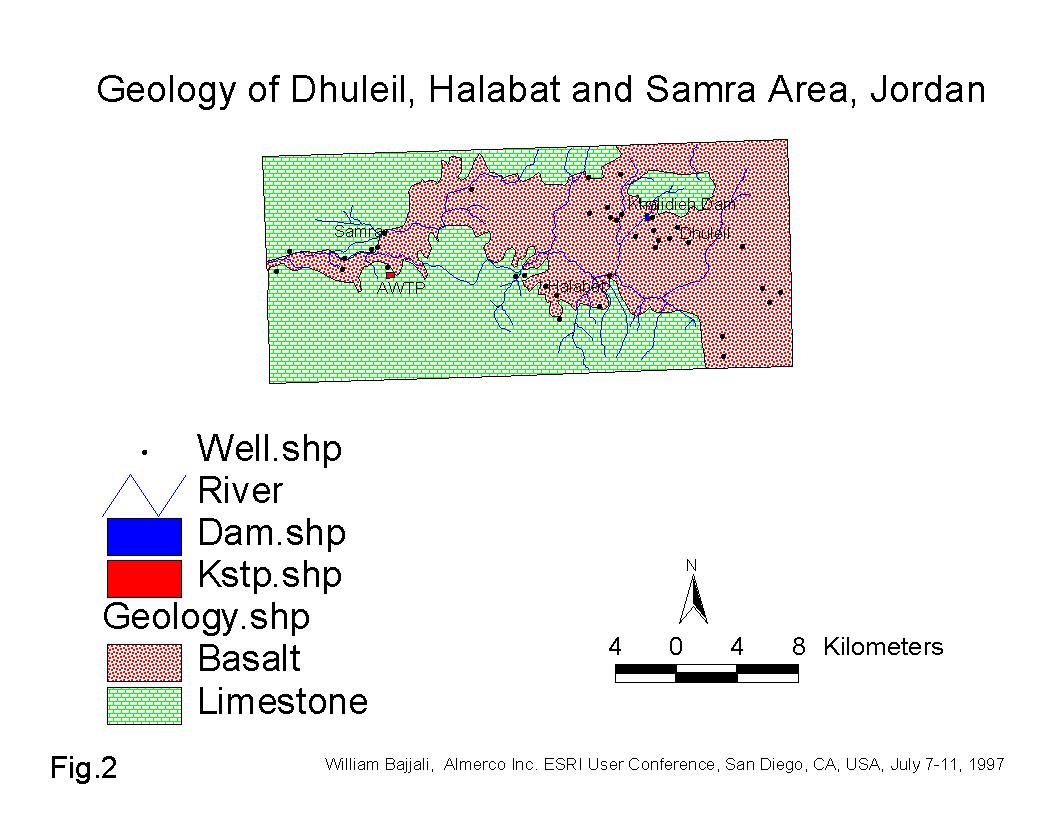
The water bearing formations are basalt and limestone. The limestone aquifer in association with the basalt aquifer which overlies it, form one hydraulic aquifer unit. The direction of the regional flow over the whole basin is from the NE Highlands of Al Druz mountain in Syria, SW towards the Sukhneh area in Jordan. In the study area, the flow moves from east (Dhuleil area) to west (Samra area).
The direction of the major structures in the area lies NW-SE, and basalt has filled a major synclinal structure having a pitch to ESE. The structure in the area influences the permeability and groundwater velocity of the aquifer system.
Basic Objectives
-
The main objectives of this study are to:
- integrate all the available chemical and isotope data into the GIS environment
- characterize the contamination and water quality of groundwater using the GIS system ArcView and Spatial Analyst
- characterize the hydrochemistry of groundwater and determine reasons for the build up of salinity in certain wells throughout the area.
GIS Approach
-
The geology of the study area was digitized in DAK and transformed to a shape file. The environmental isotope and chemical data were georeferenced and integrated into ArcView. Salinity as represented by the total dissolved solids (TDS), along with nitrate, chloride, and stable isotope of oxygen-18 were classified using the GIS system based on their concentration. The data was analyzed and interpreted using Spline method in ArcView and Spatial Analyst to produce color coded maps. The Spline method is an interpolation procedure that fits a minimum - curvature surface through the input concentration of each well. The output result is calculated through a mathematical function that includes each well and a specified number of nearest input wells.
Hydrochemistry of the Groundwaters in the Three Areas
-
For the purpose of this study a total of 37 wells were sampled and analyzed for chemistry and environmental isotope. Table 1 shows the parameters used in this study; TDS, major cations (Ca2+, Mg2+, Na+, K+), anions (Cl-, SO42-, HCO3-, NO3-), stable isotopes (18O, 2H), and radioactive isotope (3H).
Table No.1 Chemical and Environmental Isotopes Data from groundwater in the Study Area
| LON | LAT | Well No. | T | 18O | 2H | E.C | TDS | Ph | Ca2+ | Mg2+ | Na+ | K+ | Cl- | SO42- | HCO3- | NO3- |
| T.U. | o/oo | o/oo | mm/cm | ppm | meq/l | meq/l | meq/l | meq/l | meq/l | meq/l | meq/l | ppm | ||||
| 36.3504 | 32.14153 | Dhuleil-1 | 2 | -5.6 | -28.1 | 2 | 1307 | 7.8 | 4.6 | 7.4 | 7 | 0.3 | 13.3 | 3.4 | 1.6 | 50.5 |
| 36.20948 | 32.16742 | Dhuleil-2 | 0 | -7.3 | -41.2 | 2.6 | 1670 | 7.3 | 6.2 | 6.8 | 12.8 | 0.4 | 15.8 | 7.2 | 3 | 3.4 |
| 36.30239 | 32.15433 | Dhuleil-3 | 2.2 | -6.1 | -30.1 | 6.4 | 4160 | 7.2 | 16.8 | 26.5 | 25.1 | 0.5 | 50.6 | 14.8 | 1.2 | 119 |
| 36.31179 | 32.17359 | Dhuleil-4 | 0.8 | -6.3 | -32.2 | 1.7 | 1056 | 7.5 | 3.2 | 6.4 | 5.7 | 0.1 | 10.1 | 2 | 2.8 | 36.7 |
| 36.31186 | 32.14993 | Dhuleil-5 | 1.2 | -6.3 | -30.1 | 3 | 1950 | 7.7 | 7.4 | 11.7 | 10.6 | 0.4 | 21.8 | 6 | 1.1 | 76.4 |
| 36.30394 | 32.14875 | Dhuleil-6 | 0.1 | -6 | -30.1 | 3.3 | 2145 | 7.5 | 7.6 | 11.8 | 11 | 0.4 | 23.5 | 4.8 | 1.2 | 85.2 |
| 36.28983 | 32.15106 | Dhuleil-7 | 0.6 | -5.9 | -32.2 | 6.1 | 3965 | 7.6 | 15.4 | 23.9 | 24.8 | 0.6 | 46.6 | 14.6 | 1.3 | 145 |
| 36.33376 | 32.14023 | Dhuleil-8 | 0 | -5.9 | -32.1 | 3 | 1950 | 7.6 | 12 | 8.1 | 8.9 | 0.4 | 22.1 | 5.2 | 1.1 | 85 |
| 36.34433 | 32.1354 | Dhuleil-9 | 0 | -6.4 | -32.8 | 2.9 | 1911 | 7.7 | 8.8 | 11.7 | 8.4 | 0.4 | 22.2 | 4.5 | 1.1 | 88 |
| 36.32123 | 32.13672 | Dhuleil-10 | 0 | -6.1 | -30.5 | 3.1 | 2015 | 7.5 | 8.8 | 12.6 | 9.6 | 0.5 | 24.2 | 5 | 1 | 94.3 |
| 36.33443 | 32.13018 | Dhuleil-11 | 0 | -6 | -30.7 | 5.9 | 3835 | 7.7 | 16.9 | 22.9 | 24.9 | 0.6 | 47.2 | 15 | 0.9 | 142 |
| 36.35763 | 32.13244 | Dhuleil-12 | 0 | -6.4 | -33.2 | 3 | 1950 | 7.7 | 8.8 | 12 | 8.9 | 0.4 | 22.8 | 4.6 | 1.1 | 97.2 |
| 36.30855 | 32.14686 | Dhuleil-13 | 0.1 | -6.3 | -32.7 | 2.9 | 1872 | 7.8 | 7.5 | 11.3 | 9.7 | 0.4 | 21.4 | 4.8 | 1.1 | 80.6 |
| 36.33655 | 32.13442 | Dhuleil-14 | 0 | -6.2 | -30.4 | 3.9 | 2535 | 7.5 | 11.5 | 16.3 | 11 | 0.5 | 30.2 | 6.5 | 1 | 116 |
| 36.39512 | 32.12896 | Dhuleil-15 | 2.7 | -6 | -29.6 | 1.5 | 960 | 7.8 | 3.2 | 5.3 | 5.9 | 0.3 | 8.1 | 4.2 | 1.5 | 32 |
| 36.42006 | 32.10102 | Dhuleil-16 | 6.8 | -5.8 | -31.6 | 0.8 | 499 | 8.2 | 2 | 2.7 | 2.8 | 0.2 | 4.1 | 1.3 | 1.8 | 26.6 |
| 36.2895 | 32.17251 | Dhuleil-17 | 15.3 | -5.7 | -28.1 | 0.6 | 352 | 8 | 1.3 | 1.7 | 2.4 | 0.1 | 1.4 | 0.9 | 3 | 26 |
| 36.41234 | 32.0956 | Dhuleil-18 | 0.1 | -6.4 | -33.1 | 1.5 | 960 | 7.8 | 3.2 | 5.3 | 5.9 | 0.3 | 8.1 | 4.2 | 1.5 | 32 |
| 36.33329 | 32.14798 | Dhuleil-19 | 5.3 | -5.6 | -26.3 | 2 | 1326 | 7.7 | 4.8 | 7.3 | 7 | 0.2 | 13.7 | 3.7 | 1.6 | 51.3 |
| 36.40791 | 32.10409 | Dhuleil-20 | 0.6 | -6.2 | -34.6 | 3.4 | 2210 | 7.8 | 10.5 | 12.5 | 8.4 | 0.4 | 26.2 | 2.6 | 1.3 | 101 |
| 36.29447 | 32.09659 | Halabat-1 | 1.8 | -6.4 | -31.9 | 1.3 | 826 | 7.8 | 2.1 | 2.5 | 6.9 | 0.2 | 6.8 | 2.8 | 1.7 | 22.9 |
| 36.23793 | 32.11576 | Halabat-2 | 0.2 | -6.4 | -34 | 1.1 | 710 | 8 | 2.2 | 2.4 | 5.6 | 0.2 | 6.3 | 1.8 | 1.7 | 25 |
| 36.37891 | 32.07635 | Halabat-3 | 0 | -6.5 | -33.1 | 0.3 | 218 | 7.3 | 0.6 | 0.6 | 2 | 0.1 | 1.2 | 0.3 | 1.5 | 10.1 |
| 36.26508 | 32.10353 | Halabat-4 | 0 | -6.5 | -32.4 | 1.2 | 755 | 7.8 | 2.1 | 2.6 | 6.5 | 0.2 | 6.4 | 2.7 | 1.7 | 25.4 |
| 36.26642 | 32.0897 | Halabat-5 | 0 | -6.3 | -31.9 | 1.8 | 1133 | 7.7 | 3.9 | 3.8 | 8.7 | 0.2 | 11.9 | 2.7 | 1.6 | 35.8 |
| 36.37962 | 32.06461 | Halabat-6 | 0.6 | -6.5 | -33.2 | 3.6 | 2340 | 7.2 | 9.9 | 13.7 | 10.4 | 0.4 | 26.2 | 4.8 | 1.2 | 138 |
| 36.25737 | 32.10902 | Halabat-7 | 0 | -6.4 | -31 | 3.2 | 2080 | 7.7 | 5.4 | 7 | 19 | 0.2 | 20.4 | 8.1 | 1.6 | 89.4 |
| 36.24413 | 32.11586 | Halabat-8 | 0 | -7.1 | -34.6 | 0.5 | 333 | 8 | 1 | 1.3 | 2.9 | 0.1 | 1.8 | 0.9 | 2.5 | 1.9 |
| 36.30194 | 32.11437 | Halabat-9 | 0 | -6.6 | -32.4 | 0.6 | 352 | 8.2 | 0.6 | 1.1 | 3.3 | 0.2 | 2.8 | 0.4 | 2.1 | 1 |
| 36.14787 | 32.14317 | Samra-1 | 5.2 | -5.3 | -32.8 | 5.4 | 3510 | 7.2 | 22.7 | 19.4 | 11.9 | 0.3 | 42.9 | 7.3 | 2.1 | 119 |
| 36.08189 | 32.13385 | Samra-2 | 2.5 | -6 | -30.5 | 7.9 | 5135 | 7.3 | 25.4 | 28.9 | 30.5 | 0.5 | 63.3 | 17.8 | 2 | 145 |
| 36.14949 | 32.12309 | Samra-3 | 6.7 | -6.1 | -25.5 | 0.3 | 186 | 7.7 | 2 | 0.3 | 0.5 | 0.2 | 0.2 | 0.2 | 2.6 | 7.4 |
| 36.13867 | 32.13383 | Samra-4 | 6.7 | -5.6 | -28.5 | 5.7 | 3705 | 7.1 | 23.7 | 21.7 | 13.7 | 0.1 | 44.5 | 8.4 | 2.1 | 187 |
| 36.1427 | 32.13502 | Samra-5 | 3.5 | -3.8 | -25.4 | 14 | 9067 | 6.9 | 68.4 | 59.7 | 30.7 | 0.7 | 115.6 | 36.2 | 2.6 | 324 |
| 36.11961 | 32.12906 | Samra-6 | 1.1 | -6.3 | -30.5 | 2.3 | 1502 | 7.6 | 7.4 | 6.8 | 8.2 | 0.2 | 14.8 | 3.9 | 2.6 | 66.7 |
| 36.11864 | 32.1225 | Samra-7 | 1.1 | -5.9 | -31.6 | 8.2 | 5330 | 7.1 | 27.1 | 30.5 | 32.5 | 0.2 | 64.8 | 20.1 | 1.9 | 154 |
| 36.07232 | 32.12262 | Samra-8 | 3.6 | -5.6 | -29.5 | 2.6 | 1716 | 7 | 8 | 5.8 | 12.6 | 0.2 | 15 | 5.6 | 4.9 | 66 |
Salinity of Groundwater
-
The majority of the wells penetrated only the upper part of the aquifer which is a basalt formation. Basalt yields large amounts of excellent quality water suitable for various consumption uses because it is a silicate mineral and does not dissolve very fast in groundwater. Therefore, the quality of groundwater before agricultural expansion in the early sixties was excellent.
The range of salinity at the beginning of the drilling program was between 265 and 448 mg/l. The continuous extraction of groundwater for irrigation over the last 25 years has resulted in very poor water quality. The salinity of some DP-wells in the Dhuleil area increased 4 to 13 times above the initial level for the period from 1965 to 1989 (Table 2 and Fig.3). Effectively, the groundwater in certain areas changed from good, fresh quality water to undesirable brackish water. These wells are located within the agricultural land activities in the Dhuleil area.
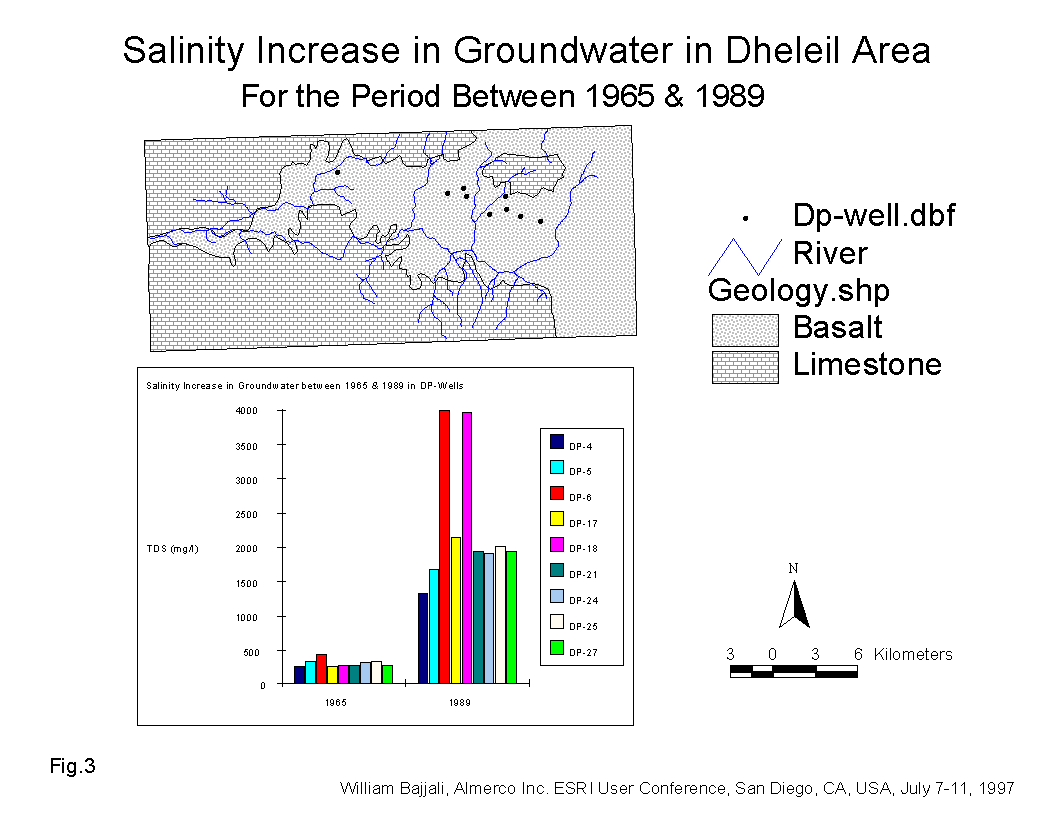
Table No.2 Salinity Increase in Groundwater from 1965 to 1989
| 1965 | 1989 | Rate of Increase | |
| Name | TDS (mg/l) | TDS (mg/l) | % |
| Dhuleil-1 | 275 | 1307 | 475 |
| Dhuleil-2 | 346 | 1670 | 483 |
| Dhuleil-3 | 448 | 4160 | 929 |
| Dhuleil-6 | 265 | 1862 | 703 |
| Dhuleil-7 | 291 | 3965 | 1363 |
| Dhuleil-8 | 288 | 1931 | 670 |
| Dhuleil-9 | 314 | 1911 | 609 |
| Dhuleil-10 | 346 | 1950 | 564 |
| Dhuleil-12 | 292 | 1950 | 668 |
The salinity of groundwater reached such elevated levels that it became undesirable not only for drinking purposes but also for irrigation use. This became a major issue and source of concern for the Water Sector in Jordan.
There are several ways in which the salinity of groundwater can be increased. One of the most important mechanisms is called cyclic salting. This is a term applied to salt input to the groundwater from precipitation. However the range of TDS in precipitation in 10 rainfall stations throughout the country ranged between 38 mg/l and 210 mg/l (Bajjali, 1990). For this reason, precipitation in the region could not be the major contributor and source of the high salt level in the local groundwater.
The second possible source of salts is rock weathering in the area. However the outcropping formations are basalt and limestone, neither of these rock types could contribute significantly to the salt accumulation in the aquifer system.
The third potential contributor to rising salt levels is the invasion of saline water from the deeper aquifer. A previous study was conducted to evaluate the salinity build up in certain wells and concluded that an invasion of saline water from the deep layer to the aquifer caused by over-pumping is the main reason for the salination problem (Nitsch, 1990). The potential of this process being relevant in this particular case is slim for the following reason. If saline water is moves from the lower layer to the upper layer due to the difference in hydraulic head caused by heavy pumping, the salinity concentration of the water in the productive wells should be more or less the same. However, this is not observed. In addition, these wells have differing types of saline water, including NaCl, CaCl2, and MgCl2 and different molal ratios of Ca/Mg, Cl/Na, Cl/Ca (Table 3).
The fourth and the most likely source of potential salinity increase is associated with local irrigation methods. It is believed that continued re-irrigation of the soil from the groundwater aquifer is causing a build up in salinity. Various techniques are used for irrigation including sprinkler and open channel. The latter makes the water subject to intensive evaporation. Irrigation water evaporating from the soil and the roots of plants, precipitates salts above and below the soil surface. Constant irrigation from the groundwater of the same area continues to dissolve the accumulated salts from the soil. The salt is flushed and washed out repeatedly from the soil causing infiltration of the solute into the subsurface thereby increasing the salinity of groundwater. This practice has continued for more than two decades near the productive wells in the area.
The white color of the soil surface throughout the study area is evidence of salt accumulation. Soil analyses in the mid sixties (the beginning of the agricultural development), indicates that NaCl and Na2CO3 are the major salts in the soil (Nitsch, 1990).
The dissolution of the mineral salts is achieved by chemical attack. The calculated log PCO2 value (Table 3) of the groundwater wells exceeds the PCO2 of the air which is only 10-3.5 (Freeze and Cherry, 1979). This allows the irrigation water to dissolve the highly soluble minerals present in the soil.
The chloride (Cl-) anion is the major dissolved element whose concentration has increased many times in comparison to the other negative and positively charged ions dissolved in the groundwater. Chloride is generally conservative in groundwater due to its highly solubility and because it is not subject to exchange or adsorption during groundwater flow in the aquifer system. The high concentration of Cl- in groundwater is the result of dissolution of Cl- type minerals accumulated either in the soil or deposited in the subsurface geological formation. Figure 4, shows a general increase in Cl- concentration along the flow path from east to west in the study area. The highest concentration was recorded for wells located downstream of the sewage treatment plant in the Samra area and around agricultural land in Dhuleil area. The range of Cl- concentration in the groundwater is between 5 and 4000 mg/l (Fig.4). The highest Cl-concentration (2000 - 4000 mg/l) was found in the Samra area wells located nearby and downstream of the AWTP. A range of between 1000 and 2000 mg/l was found in the Dhuleil, Halabat, and Samra area wells where agricultural activities are widely spread. The dissolution of highly soluble minerals such as CaCl2, MgCl2, and NaCl are responsible mainly for build up of the salinity in the groundwater. The solubility of these salts increases as temperatures rise. For example if CaCl2, MgCl2, and Na2SO4 are precipitated from the NaCl and CaSO4 solution; their solubility expressed in g/kg of solution in 20 oC are 427, 351, and 160 respectively (Dan Yaron, 1981).
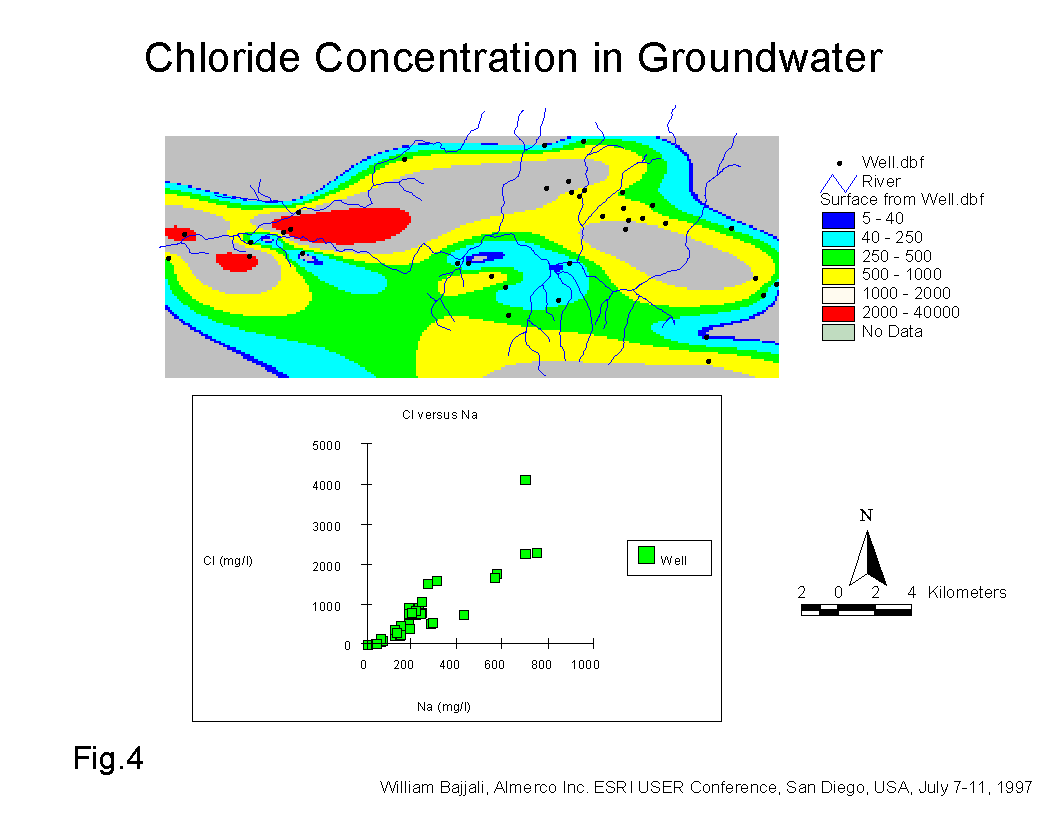
Figure 4 also shows a plot of Na+ concentration versus Cl- concentration in mg/l for all the groundwater samples. The low slope of 0.21 and R2 of 0.78 indicates that the ion exchange reaction keeps the chloride concentration constant and decreases the sodium in the solute in the saturated zone. Smectite and smectite/illite type clay minerals are common in the top soil in the vicinity of the study area (Bajjali, 1988). The high molal ratio 1.23 to 3.76 of Cl/Na for the Samra and Dhuleil areas (Table 3) are the result of cations absorption by these clay minerals.
Salinity is also significantly increased in the Halabat and Samra areas. In the Hallabat, like in the Dhuleil area, the salinity increase is due to agricultural activities. In the Samra area a wide range of TDS concentration (between 186 mg/l and 5000 mg/l) was recorded in the groundwater wells. The low TDS value represents a collective rain water well located upstream of AWTP while the high concentration represents shallow wells located 2 km downstream of AWTP. The concentration of one of the wells (Samra-5) located in close proximity to the sewage treatment plant reached up to 9000 mg/l. In this case, three sources could simultaneously be responsible for increasing the salinity. The first source could come from the direct infiltration of effluent from the treated waste water (AWTP) discharging into a perennial stream called Wadi Dhuleil. The hydrogeological setting of the aquifer system downstream of the AWTP allows the infiltration to occur. The upper part of the shallow aquifer consists of alluvium which is highly permeable material. The second possible source, like the Dhuleil and Halabat areas is the dissolution of the accumulated salts in the soil by the irrigation water. The third source is due to direct evaporation from the water table (approximately 10 m) of the shallow aquifer.
Table No.3 Log PCO2 and cations Anions Ratio of Some Wells in the Study Area
| Name | LogPCO2 | Ca/Mg | Cl/Ca | Cl/Na |
| Dhuleil-1 | 0.0018 | 0.66 | 5.68 | 1.95 |
| Dhuleil-2 | 0.0078 | 0.91 | 5.09 | 1.23 |
| Dhuleil-17 | 0.0072 | 0.76 | 2.07 | 0.56 |
| Dhuleil-20 | 0.0019 | 0.76 | 5.27 | 2.57 |
| Halabat-8 | 0.0062 | 0.58 | 8.68 | 0.84 |
| Halabat-9 | 0.0066 | 0.77 | 3.60 | 0.62 |
| Samra-1 | 0.0060 | 1.17 | 3.77 | 3.60 |
| Samra-2 | 0.0043 | 0.88 | 4.98 | 2.07 |
| Samra-5 | 0.0137 | 1.14 | 3.88 | 3.76 |
| Samra-6 | 0.0037 | 1.08 | 4.00 | 1.80 |
Nitrate in Groundwater
-
Nitrate is a another chemical parameter which is found in high concentrations in some wells throughout the study area. Nitrate is the most available indicator of organic (sewage and manure) and inorganic (artificial fertilizer) sources of contamination. Nitrate is easily dissolved in groundwater, is very mobile in subsurface flows and is spread very quickly through the fracture subsurface media. The nitrate concentration in groundwater throughout the entire study area has increased beyond the level of natural abundance of nitrate in groundwater which is 0.1 - 10 ppm (Cherry and Freeze, 1979). Nitrate levels in the Samra, Halabat, and Dhuleil water wells have increased by 74%, 33%, and 57% respectively above the World Health Organization's (WHO) standard of 45 mg/l (WHO, 1984).
Chemical fertilizers, in particular Urea (nitrogen compose 46% of its content) are used extensively and without control in the Dhuleil area to increase soil fertility and crop yields. This results in very high nitrate content in the saline DP-wells in the Dhuleil area.
The most noticeable increase in nitrate is observed in wells in the Samra area especially downstream of AWTP (Fig.5). Omar Tilawi well recorded a nitrate concentration of 324 ppm. This high concentration is due to the sewage effluent of AWTP in Wadi Dhuleil which is high in ammonium (NH4) concentration (Saqqar, 1989). Through the process of nitrification the NH4 is converted to NO3 by oxidation. This process normally occurs above the water table, generally in the soil zone where the organic matter and oxygen are abundant (Cherry and Freeze, 1979).
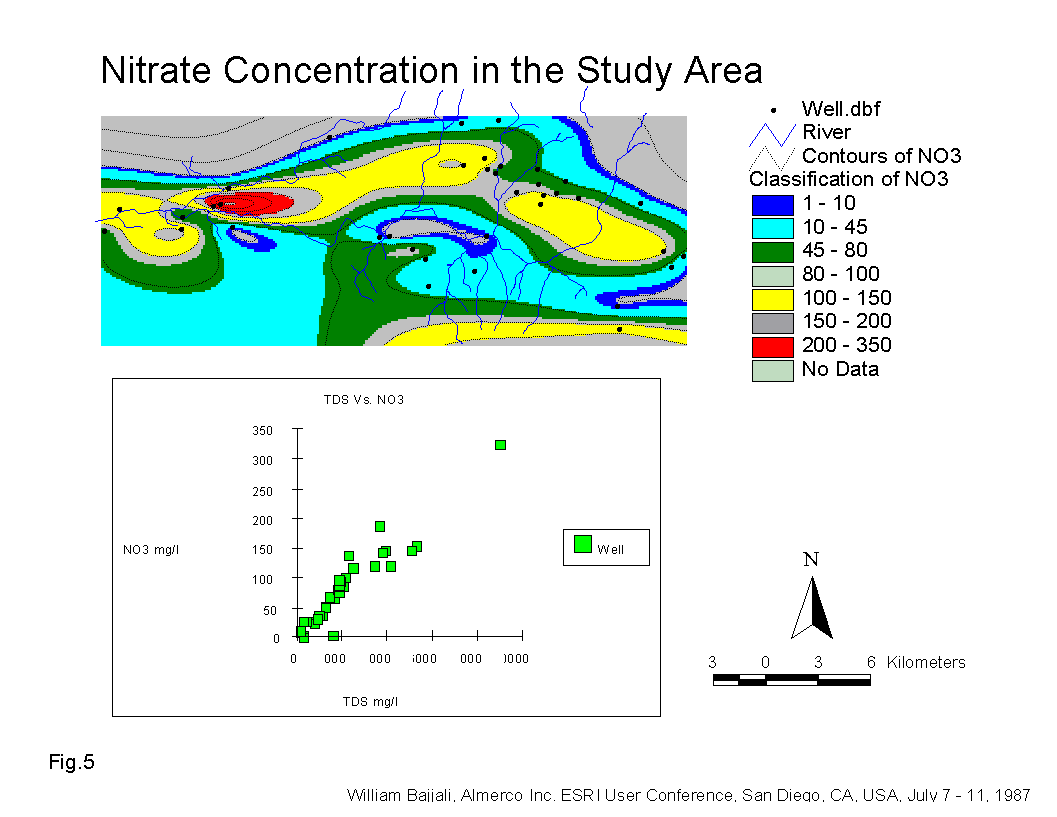
Fig.5 shows that the nitrate concentration is correlated strongly (R2 = 0.89) with the salinity of the groundwater. This is another indication that the source of contamination originates on the surface and that it is responsible for increasing the salinity in the study area. .
Environmental Stable and Radioactive Isotopes
-
The stable isotope data for all groundwater in conjunction with the rain in Amman (weighted mean value for the period of 1987-1989) are plotted on a delta 2H and delta 18O diagram (Fig.6).
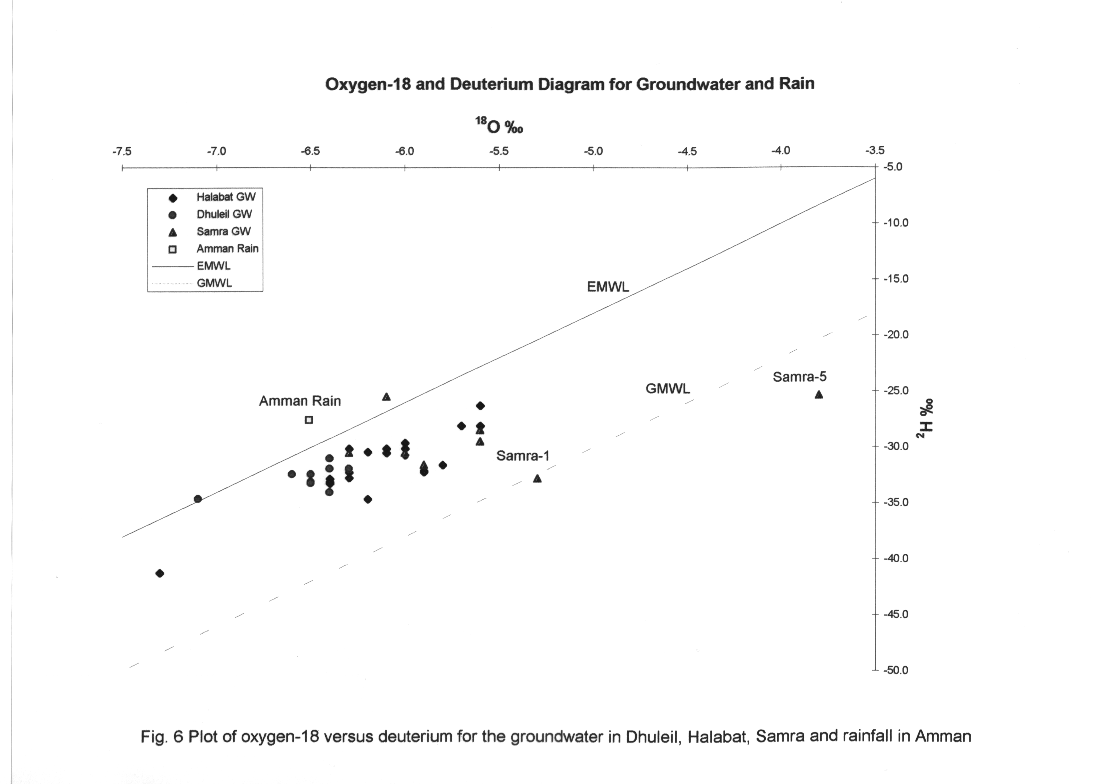
These data show that the groundwater in the area is largely associated with the Eastern Meteoric Water Line (EMWL) defined by (Gat and Carmi, 1970), indicating recharge under the climate regime which predominates today in Jordan. A close look at the isotopic composition of all the data indicates that there is at least a 3 o/oo and 15 o/oo range in both delta 18O and delta 2H. From Fig.6 we notice the following: Firstly some well samples plot along an evaporation line originating from the EMWL.
The location of the isotopic composition of Dhuleil groundwater along the EMWL is much more depleted than the weighted mean value of isotopic composition of Amman Rain, where the altitude of the Amman rainfall station is 900 m. This suggests that the recharge of the groundwater originates from a greater elevation than that of Amman.
Secondly, a wide variation in isotopic composition is observed in the water samples of the Dhuleil and Halabat areas. It is possible that this variation in isotopes is due to stratification of the groundwater. The basalt aquifer is divided by six clay layers, so every layer represents a separate path for the groundwater from the recharge area or from the return flow above the study area to the point where the water is captured by a drilling well. Heavy pumping in the summer time can also create a mixing condition of different layers permeated by the groundwater.
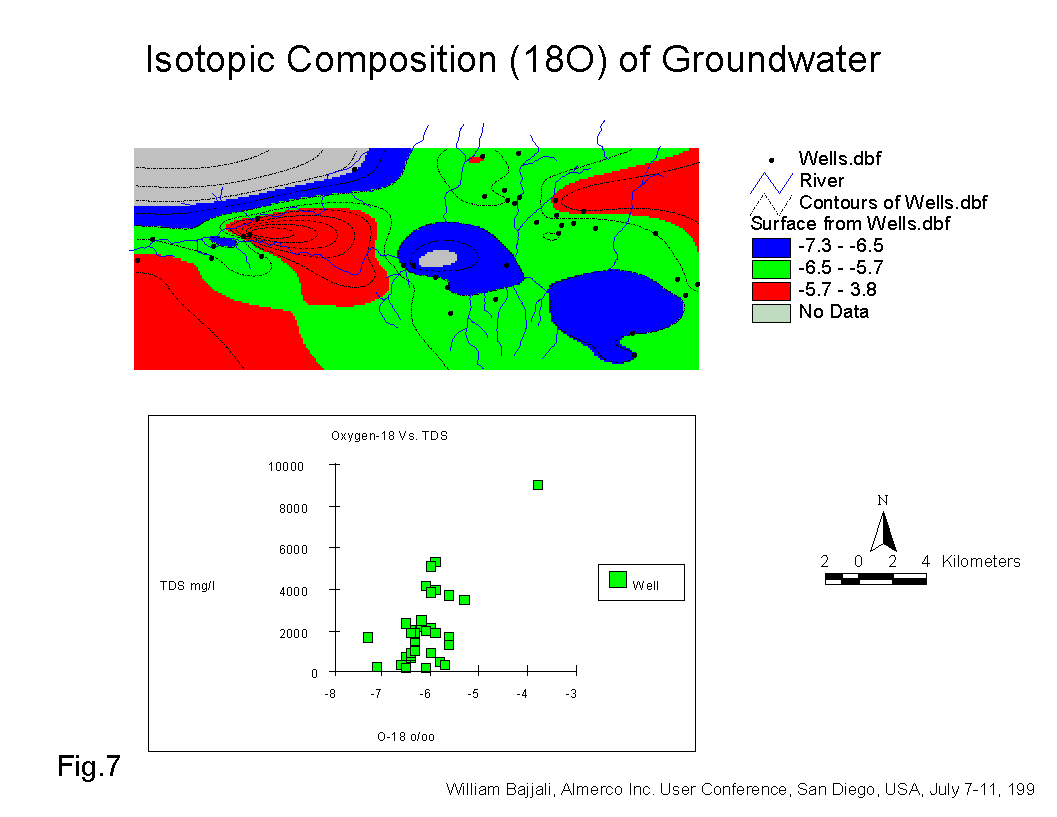
Thirdly, the return flow from irrigation and the effluent of sewage from AWTP in Wadi Dhuleil is subject to evaporation before infiltration back to the aquifer. The isotopic composition of the wells in the Samra area is completely distinct and more enriched in heavy isotope. Broad variation was found for wells located downstream of AWTP, indicating that the water infiltrating into the aquifer was subject to extensive evaporation before infiltration. Red color (Fig. 7), shows that the most enriched groundwater in delta 18O is located in the Samra and Dhuleil areas (red color).
The tritium concentration in the groundwater enables us to determine when this groundwater was recharged; i.e. pre or post bomb test held in 1952. A tritium concentration in excess of 2�1 Tritium Unit (T.U), indicates that the well was recharged after 1952. Some of the Samra wells show high concentrations of tritium. This could result from several factors: Firstly, the waste water in AWTP originates from the Amman Zarqa aquifer, where the groundwater is highly tritiated, and its concentration ranges from 2.6 to 14.7 T.U (Bajjali, 1990). The effluent of the tritiated water in Wadi Dhuleil shows a fluctuating concentration of tritium over time. The infiltration of the treated waste water from Wadi Dhuleil into the shallow aquifer increased the tritium level in the wells located nearby and downstream of the AWTP. This is clearly observed in the wells such as Samra-5 and Samra-1. Secondly, a high tritium concentration (5.3 T.U) was found in the Dhuleil observation well located 545 meters from the Khalideh dam. This dam receives its water from flooding and surface runoff where the tritium concentration is in the range of 10 T.U. In addition, the dam's water shows a wide range of delta 18O (-3.3 o/oo and -10.8 o/oo) over time. The most depleted value was recorded after an intense rainfall in the area, while the most enriched value was noted after several months of evaporation. Taking into consideration that the isotopic composition of the Dhuleil observation well (Dhuleil-19) is slightly enriched in delta 18O (-5.5 o/oo, less saline (1326 mg/l), higher tritium concentration (5.3 T.U.) and is closer to the dam than the other wells in Dhuleil area, we can conclude that the Khalideh dam acts as a direct recharge source to the groundwater in the area.
Conclusions
-
The elevated salinity in groundwater wells is due to the return flow from the agricultural activities and infiltration of treatedsewage water into the groundwater.
The saline return flow and the effluent of AWSP progressively deteriorated the quality of the groundwater. The evaporated irrigation water in the soil and around the roots of plants precipitates salts in the soil. Continuous irrigation using the same groundwater continues to dissolve the accumulated salts in the soil which then on flushes into the subsurface thereby progressively increasing the groundwater salinity.
The high nitrate concentration in groundwater rises to a very high value throughout the study area. The origin of NO3 in the groundwater is the inorganic fertilizer and the effluent of treated sewage from the AWTP.
The stable isotopes (18O and 2H) show that the water is of meteoric origin affected by the Mediterranean Sea air moisture. The recharge area of the study area originates at altitudes greater than 900 m. The wide scatter of the isotope composition is due to an evaporation effect. The man made radioactive tritium found in the shallow wells in the Samra and Dhuleil areas is evidence of local recharge.
The Khalideh dam serves as an artificial recharge during the winter season replenishing the surrounding groundwater wells, thus improving their quality.
References
-
1. Bajjali, W., 1988. Clay Mineralogy in the Industrial Awajan Area. Internal Report, Water Authority of Jordan. Amman, Jordan.
2.. Bajjali, W., 1990. Isotopic and Hydrochemical Characteristics of Precipitation in Jordan. M.Sc. Thesis. Jordan University, Amman-Jordan.
3. Bajjali, W., 1990. Hydrochemical and Isotopic Characteristic of Groundwater in Amman-Zarqa Area. Internal Report, Water Authority of Jordan, Amman-Jordan.
4. Dan yaron, 1981. Salinity in Irrigation and water Resources Marcel Deker Inc. New Yourk.
5. Freeze and Cherry, 1979. Groundwater. Prentice-Hall Inc., Englewood Cliffs, New Jersey, 07632.
6. Gat, J., and Carmi, I., 1970. Evaluation of the Isotopic Composition of Atmospheric Waters in the Mediterranean Sea Area. J. Geophys. Res., 75: 3039-3078.
7. Nitsch, M., 1990. Soil Salinization in the Wadi Dhuleil and Wadi Arja Irrigation Project. Hashemite Kingdom of Jordan. Hannover, February, 1990.
8. Saqqar, M., Matar, A., and Bajjali, W., 1989. Performance Evaluation of Wastewater Treatment Plants in Jordan and their effect on Groundwater, Internal Report, Water Authority of Jordan, Amman, Jordan
9. Water Authority of Jordan, Data-files.
10. World Health Organization, 1984. Guideline for Drinking Water Quality Volume No.1 Recommendation. Geneva.
-
William Bajjali, Ph.D.
Director of Geomatics,Almerco inc.
1695, Atmec street, Unit 8
Gatineau, Quebec
Canada
J8P 7G7
Tel: (819) 669-3170
Fax: (819) 669-1922
bajjali@almerco.ca
www.almerco.ca/bajjali.html