Abstract
Locusts and grasshoppers are serious agricultural pests that can transcend
political boundaries. Chemical pesticides are usually used to control locust
and grasshopper populations, but with increased environmental concerns,
alternative natural biological control agents are increasingly being used.
The entomopathogenic fungus, Metarhizium anisopliae var acridum,
can be used as a biopesticide to control locusts and grasshopper pests.
However, temperature is a key factor governing the virulence of the pathogen,
resulting in variable performance of the biopesticide in the field; sometimes
control is very rapid, other times effective control may not be achieved.
This has led to the development of a pathogen-performance model that can
accurately predict the speed of kill in a field environment. Here we use
a GIS to model and investigate the spatial and temporal variation in pathogen
performance against the Moroccan locust in Spain. The implication of these
results are discussed in terms of strategic implementation and use of this
biopesticide.
Introduction
Locusts and grasshoppers are serious agricultural pests causing extensive
damage to food crops and pasture throughout the world. Chemical pesticides
are used to control outbreaks. More recently, concerns over the environmental
and health effects of chemicals (e.g. toxicity to non-target species (Tingle,
1996) and humans (Pretty, 1996)) has led to an increase in the use of more
environmentally benign methods of locust control. One control agent, the
entomopathogenic fungus, Metarhizium anisopliae var acridum,
has received considerable attention over the last 15 years as a viable
biopesticide alternative to chemicals. The fungus is highly specific to
the Acrididae, the family of short-horned grasshoppers to which the majority
of economically important grasshoppers and locusts belong. It can be mass-produced
relatively easily on artificial solid substrates and when formulated in
oil, can be applied under a range of environmental conditions using current
chemical application technology. The biopesticide resulting from this research
and development has been registered for use in parts of Africa (Green Muscle®),
is currently undergoing registration in Australia (Green Guard®) and
is being tested and developed in a number of other locust-affected countries
around the world (Thomas et al., 2000).
While the details mentioned above imply that M. anisopliae can be a successful biological control agent for locust and grasshoppers, the effectiveness of the pathogen, as with many biocontrol agents, can be highly variable (Bateman & Thomas, 1996; Lomer et al., 1999). Without understanding and being able to predict this variability, confidence and widespread uptake of this green alternative may be affected.
Recent research has identified that the key constraint, affecting efficacy is temperature. Metarhizium anisopliae develops and kills its host most rapidly at about 30oC (Arthurs & Thomas, 2001). It shows no development below about 10oC or above 40oC (Thomas & Jenkins, 1997; Ouedraogo et al., 1997). Moreover, many locusts and grasshoppers are active behavioural thermoregulators. That is, via a combination of habitat choice and body postures they balance heat gain and heat loss to maintain a preferred body temperature for large parts of the day (Chappell & Whitman, 1990). For many species in arid and semi-arid environments, the preferred body temperature is around 38oC, which severely limits pathogen growth. In addition, infected locusts and grasshoppers have also been shown to mount a defense response, raising body temperature further through behavioural fever to around 42oC (Blanford et al., 1998; Blanford & Thomas, 1999). Thus, body temperature of the host (both direct temperature and the temperature-mediated defense response) critically determines the rate of pathogen development and hence, speed of kill. This can lead to considerable variation in mortality rates across time and space. For example, in environments with warms nights around 20-25oC (i.e. conducive for M. anisopliae growth), combined with relatively short days (limiting the number of thermoregulation hours by the locust), 50-100% mortality can be achieved in less than 15 days (e.g. Langewald et al., 1999; Hunter et al., 2001). On the other hand, in regions where day length is longer (providing considerable thermoregulatory opportunity for the host) and nights are cold (perhaps 5-15oC at which pathogen growth is very slow), 50% mortality may take longer than 35 days with some individuals surviving well into reproductive maturity (Arthurs & Thomas, 2000). Therefore the key to using the biopesticide effectively is to understand the dynamic interaction between the host and pathogen in the field environment where it will be used (Blanford & Thomas, 1999).
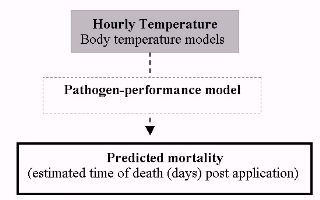
Thus, by providing end-user support to determine when and where the biopesticide is most effective, we have developed a decision-making tool that predicts pathogen-performance across space and time. The model estimates time to death of locusts treated with the biopesticide based on the interaction between the host and pathogen, driven by temperature mediated by the thermal biology of the host (thermoregulation) (see Figure 1). Model accuracy, in comparison to empirical field trial data for a number of different species in a diverse range of habitats, has been found to be very good.
Figure 1: Illustration of the key model components predicting pathogen-performance under field conditions

Several studies have successfully mapped the abundance of vector-borne
diseases and/or insect pest incidences using environmental data through
satellite images. For example, Rogers and Randolph (2001) illustrated changes
in malaria distribution with climate change. Similarly, Voss and Dreiser
(1994) successfully mapped locust habitats and the FAO have developed a
locust forecasting and monitoring system in a GIS to determine when and
where the desert locust populations will occur (Healey et al., 1996).
These studies have mainly used vegetation classification or normalized
differential vegetation index as proxies to temperature and soil moisture.
Since the pathogen-performance model is based on hourly pathogen development,
the temporal scale at which these remotely sensed data are captured is
too coarse a measure to provide accurate estimates. Instead, we coupled
the pathogen-performance model with environmental data in a geographic
information system (GIS) to investigate spatial and temporal performance
of the biopesticide. Here we illustrate how the performance of the biopesticide
can change spatially and temporally within a season using, as an example,
the Moroccan locust, Dociostaurus marocannus, in Spain.
Methods
Study species and study site
The Moroccan locust, Dociostaurus maroccanus, has been recorded
as an important pest of pasture and crops in Spain for several centuries.
In excess of 500,000 ha-1 are affected in the provinces of Badajoz,
Ciudad Real, Almeria and Zaragoza. Through an EU-funded project, we are
currently investigating the use of M. anisopliae for control of
D.
maroccanus, at a field site (Castuera) in Spain. Because the key outbreak
areas of D. maroccanus occur in four geographically discrete regions
(Castuera, Ciudad Real, Zaragoza and Almeria) there is the possibility
that good control in one area may not be mirrored in any of the other areas.
Thus, for the purpose of this study, we are interested in modeling efficacy
of the pathogen against the Moroccan locust in these four regions.
Data
Hourly temperature data are important for estimating locust body temperature
and pathogen growth. Daily minimum and maximum temperature data collected
at meteorological stations throughout Spain for the months of April and
May 2000, were obtained from the Meteorological Office, UK, and used to
create hourly temperature surfaces. Minimum and maximum temperature surfaces
were created with a 1-km cell resolution, and an inverse square distance
interpolate using the five nearest stations. Temperature surfaces were
standardised for elevation (DEM downloaded from the USGS website (USGS,
1999)) using a lapse rate model (Jones & Gladkov, 1999). Since temperatures
can fluctuate throughout a 24-hour period, the model requires hourly temperature
estimates. Minimum and maximum temperature surfaces were substituted into
the sine-exponential model (Patron & Logan, 1981) to estimate hourly
temperatures. Hourly locust body temperatures (Tb) were then calculated
from the ambient temperatures (Ta) using the logistic regression model
proposed by Kemp (1986). Because maximum temperatures collected at the
field site can be 5-10 °C higher than those recorded at the meteorological
station, the body temperature model for D. maroccanus was derived
using simulated temperature estimates, for each minute, based on the temperatures
recorded at the meteorological station. Parameters for D. maroccanus
were
estimated using the following equation:
Final mapped outputs illustrate the predicted number of days post application required to achieve 90% mortality in the field during the months of April and May. Further details of these methodologies are presented in Klass et al. (in prep)
Results
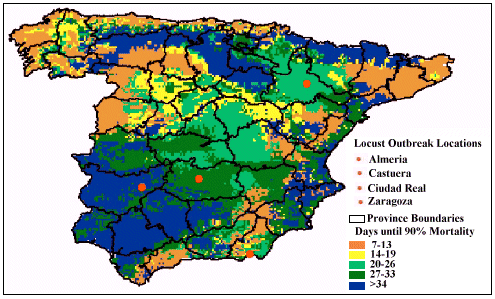
Figure 2 illustrates that pathogen-performance was predicted to have
a variable speed of kill throughout Spain taking between 7 and 34+ days
to achieve 90% mortality. During April, predicted mortality was fastest
(< 14 days) along the eastern coast and the Guadalquivir valley in the
south, and was the least effective (taking in excess of 34 days) in north
and central Spain.
When pathogen-performance was investigated a month later, predicted mortality changed considerably. Figure 3 shows that the virulence of the pathogen improved in the central and northern regions of Spain. For example, in the non-mountainous regions, mortality was predicted to occur between 14 and 33 days instead of the 34+ days predicted for the previous month. Similarly, regions in southern Spain where efficacy was very good in April (i.e. taking less than 19 days), now also showed a slower rate of kill (34+ days).
Figure 2: Predicted time taken (in days) to achieve 90% mortality
against the Moroccan locust in Spain, when sprayed by the pathogen-based
biopesticide during the month of April, 2000
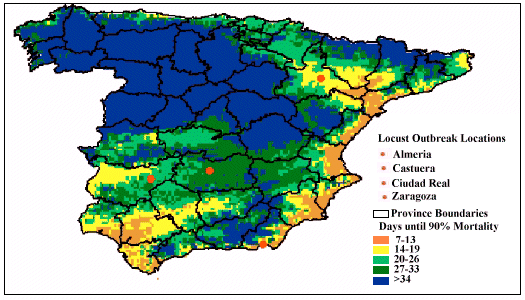
Figure 3: Predicted speed of kill, by the pathogen-based biopesticide,
against the Moroccan locust throughout Spain during May, 2000
How does pathogen-performance affect control of locusts at the key
sites throughout Spain?
Model predictions indicated that pathogen efficacy varied between locust
outbreak areas and the two months investigated (see Table 1). During April,
mortality was predicted to take the longest at Ciudad Real (27-33 days),
followed by Castuera (20-26 days), and then Zaragoza and Almeria (where
90% mortality was predicted to take less than 19 days). In May, performance
remained the same at Ciudad Real, but changed for the remaining three sites.
Mortality was found to be slower for both Castuera (> 34 days) and Zaragoza
(20-26 days), while results showed that pathogen performance became more
variable at Almeria, ranging between 14 and 26 days.
Table 1: Summary of changes in pathogen performance at the four major locust outbreak areas in Spain between April and May, 2000
| Locust Outbreak Areas | April | May |
| Almeria | 7-13 | 14-26 |
| Castuera | 20-26 | >34 |
| Ciudad Real | 27-33 | 27-33 |
| Zaragoza | 14-19 | 20-26 |
Conclusion
We have illustrated how a model describing the biological interaction
between a pathogen and its host can be combined with environmental data
to predict both spatial and temporal changes in virulence of a pathogen
within a country. Results showed that the speed of kill by M. anisopliae
can vary between 7 and 34+ days between discrete locust population areas
within a country during a month. Furthermore, the rate of mortality can
be reduced by 8 to 10 days between successive months. The alteration in
pathogen-performance can have profound effects in achieving effective control
and on the use of the biopesticide as an alternative to chemicals for control
of locusts and grasshoppers.
Incorporating the pathogen-performance model in a GIS system allows us to clearly illustrate changes in pathogen-performance both in space and time. Using historical data we hope to use the model to develop efficacy maps defining, on average, the most appropriate sites and times for utilizing the biopesticide. In addition, using real-time data collected from local meteorological stations, it could also allow locust control officers to monitor expected mortality rates concurrently with ongoing control efforts.
Acknowledgements
This work is supported by the European Commission through the
Quality of Life and Management of Living Resources Programme implemented
under the Fifth Framework Programme - contract number QLRT-1999-01118 -
Protecting Biodiversity through the Development of Environmentally Sustainable
Locust and Grasshopper Control in Europe (ESLOCO). A special thanks
to S. Elliot for providing some useful comments to the final draft of this
paper and to A. Alleyne for programming the astromical algorthims that
were used to determine hourly temperature at any given time throughout
the year in space.
References
Arthurs, S. & M. B. Thomas (2000) Effects of a mycoinsecticide
on feeding and fecundity of the brown locust Locustana pardalina.
Biocontrol
Science and Technology, 10:321-329.
Arthurs, S. & M. B. Thomas (2001) Effects of dose, pre-mortem host incubation temperature and thermal behaviour on host mortality, mycosis and sporulation of Metarhizium anisopliae var acridum in Schistocerca gregaria. Biocontrol Science and Technology,11(3):411-420.
Bateman, R. P. & M. B. Thomas (1996) Pathogen application against locusts and grasshoppers: insecticide or biological control? Antenna,20:10-15.
Blanford, S. & M. B. Thomas (1999). Host thermal biology: the key to understanding insect-pathogen interactions and microbial pest control? Agricultural and Forest Entomology, 1:195-202.
Blanford, S., M. B. Thomas, and J. Langewald, (1998) Behavioural fever in the Senegalese grasshopper, Oedaleus senegalensis, and its implications for biological control using pathogens. Ecological Entomology, 23:9-14.
Chappell, M. A. & Whitman, D. A. (1990) Grasshopper thermoregulation. In: Biology of Grasshoppers (eds. R.F. Chapman & A. Joern) Wiley Interscience, New York, pp. 143-172.
Healey, R. G., S. G. Robertson, J. I. Magor, J. Pender and K. Cressman, (1996) A GIS for desert locust forecasting and monitoring, International Journal of Geographical Information Systems, 10(1):117-136.
Hunter, D. M., R. J. Milner, and P.A. Spurgin (2001) Aerial treatment of the Australian plague locust, Chortoicetes terminifera (Orthoptera : Acrididae) with Metarhizium anisopliae (Deuteromycotina : Hyphomycetes). Bulletin of Entomological Research, 91 (2):93-99.
Jones, P. G. & A. Gladkov (1999) FloraMap: A computer tool for predicting the distribution of plants and other organisms in the wild (Version 1). Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia.
Kemp, W. P. (1986) Thermoregulation in three rangeland grasshoppers. Canadian Entomologist, 118:335-343.
Langewald, J., Z. Ouambama, A. Mamadou, R. Peveling, I. Stolz, R. Bateman, S. Attingon, S. Blanford, S. Arthurs, and C. Lomer (1999) Comparison of an insecticide with a mycoinsecticide for the control of Oedaleus senegalensis (Orthoptera: Acrididae) and other Sahelian grasshoppers at an operational scale. Biocontrol Science and Technology, 9:199-214.
Lomer, C.J et al. (1999) Development of strategies for the incorporation of biological pesticides into the integrated management of locusts and grasshoppers. Agricultural and Forest Entomology,1:71-88.
Ouedraogo, A., J. Fargues, M. S. Goettel, and C. J. Lomer (1997) Effect of temperature on vegetative growth among isolates of Metarhizium anisopliae and M. flavoviride. Mycopathologia, 137(1):37-43.
Parton, W. J. & J. A. Logan (1981) A model for diurnal variation in soil and air temperature. Agricultural Meteorology, 23:205-216.
Pretty, J. N. (1995) Regenerating Agriculture: Policies and Practice for Sustainability and Self-reliance. Earthscan Publications Ltd., London, England. 320 pp.
Rogers, D. J. & S.E. Randolph (2001) The global spread of malaria in a future, warmer world. Science, 289:1763-1766.
Tingle, C. C. D. (1996) Sprayed barriers of diflubenzuron for control of the migratory locust (Locusta migratoria capito (Sauss)) [Orthoptera: Acrididae] in Madagascar: Short-term impact on relative abundance of terrestrial non-target invertebrates. Crop Protection,15(6):579-592.
Thomas, M. B. & N. E. Jenkins (1997) Effects of temperature on growth of Metarhizium flavoviride and virulence to the variegated grasshopper, Zonocerus variegatus. Mycological Research, 101(12):1469-1474.
Thomas, M. B., J. Klass and S. Blanford (2000) The Year of the Locust. Pesticide Outlook, pp.192-195.
USGS (1999) GTOPO30 Source Data. http://edcwww.cr.usgs.bov/landdaac/gtopo30/gifs/gt30src.gif
Voss, F. & U. Dreiser (1994) Mapping of desert locust and other migratory pests habitats using remote sensing techniques. pp:23-41. In Krall, S and Wilps, H. (eds.) 1994. New Trends in Locust Control. Deutsche Gessellschaft fur Technishche Zusammenarbeit (GTZ), Germany. pp 182.
Author Contact: justine.klass@ic.ac.uk
Population biology and biological control group
NERC Centre for Population Biology and CABI BIOSCIENCE
Imperial College, Silwood Park
Ascot, Berkshire
SL5 7PY
United Kingdom